Overview
The progression in cultured meat production now includes the use of edible shellfish-derived particles and collagen. This distinctive fusion seamlessly incorporates into the final meat product, hastening cell growth to amplify efficiency and quality. By integrating these elements, it not only reduces costs but also enhances the nutritional content, texture, and visual allure of meat alternatives. This method represents a notable advancement in sustainable options for traditional meat products, appealing to eco-conscious consumers in search of ethical and inventive dietary selections.
Introduction
The domain of cultured meat, involving the creation of meat from isolated animal cells via tissue engineering, stands as a rapidly progressing realm within modern food biotechnology. This advancement is primarily driven by the escalating concerns for animal welfare and environmental impact, coupled with the pressing need to discover sustainable food sources for our expanding global populace. The latter is expected to heighten meat consumption by 73% by 2050. Cultured meat strives to align with the nutritional profiles and sensory experience of conventional meat, presenting a hopeful resolution to the imminent food scarcity.
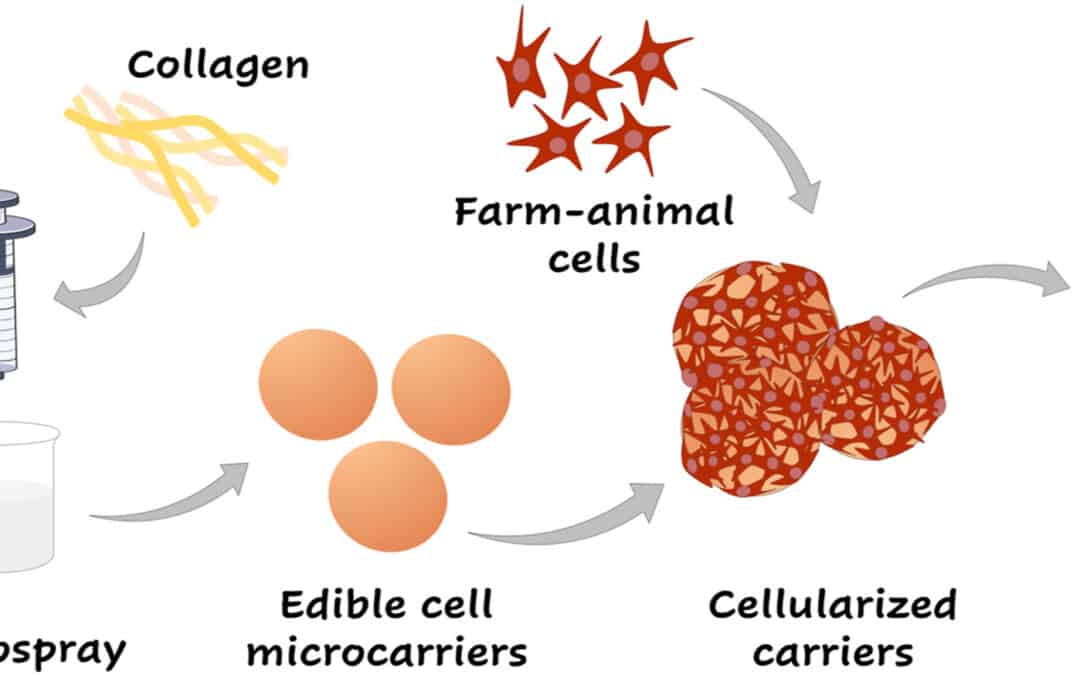
Although the concept of engineering animal tissue for food production was introduced two decades ago, the actualization of cultured meat encounters substantial, conceptual, and technological obstacles. These hurdles encompass the precise selection of cells, the management of material science elements, and the establishment of scalable culturing frameworks. The predominant technique for industrially cultivating mammalian cells at scale involves the use of suspension bioreactors that facilitate the large-scale production of suspended cells. Moreover, it is plausible to cultivate anchorage-dependent cells in suspension bioreactors through the application of cell microcarriers, on which the cells can propagate and differentiate.

Traditionally, microcarriers are crafted from synthetic materials such as glass, polystyrene, or biopolymers like dextran and Poly(lactic-co-glycolic acid), serving the purpose of expansively cultivating anchorage-dependent cells. Post-cultivation, the expanded cells undergo diverse separation methods and are harnessed for an array of biotechnological applications. In the context of cultured meat fabrication, where massive cell production is pivotal, harvested cells are generally seeded on edible scaffolds to structure a meat-like composition. This methodology aims at reducing expenses by eradicating costly harvesting stages and yield losses linked with conventional methods, while also enhancing the nutritional profile, texture, and visual appeal of the end product. Subsequent to expansion, the cell-laden microcarriers can be manipulated via physical, chemical, and enzymatic methodologies to mold them into cultured meat products like substantial cuts or patties.
The development of these edible microcarriers can build upon the existing frameworks employed in tissue engineering and cell-based treatments. Nonetheless, fabricating microcarriers for food-related purposes necessitates meticulous exclusion of toxic constituents from both the procedure and the final output.

In one study, the CellScale MicroTester G2 was employed to evaluate the mechanical properties of the polymer microcarriers produced for mass cell production. Using force-sensing micro-wire technology, the MicroTester G2 determined stress-strain properties, crucial for assessing the stability and suitability of the microcarriers for cell culture applications. This enabled researchers to characterize the resilience and structural integrity of the microcarriers, essential considerations for their effectiveness in supporting cell growth in cultured meat production.

Key points
- The introduction of edible gel particles derived from shellfish and collagen represents a significant innovation in lab-grown meat production, aiming to accelerate cell growth and improve product quality.
- Incorporating shellfish-derived particles and collagen into cultured meat production streamlines processes, reduces costs, and enhances nutritional content, texture, and visual appeal, appealing to eco-conscious consumers.
- Utilizing edible gel particles and collagen underscores a broader trend towards sustainable meat alternatives, addressing concerns for animal welfare, environmental impact, and meeting the demands of a growing global population.
What’s Next?
Curious about the MicroTester? The MicroTester has the perfect 50 micron to 5 millimeter scale needed for researchers at Georgia Tech. Their samples are not tissues, or single cells but rather micromass aggregates of stem cells. Learn how our technology surpasses Instron machines or atomic force microscopy to get the mechanical properties of their samples.